by Randal Chinnock
Slide 8 of 12
PREVIOUS SLIDE – – – NEXT SLIDE
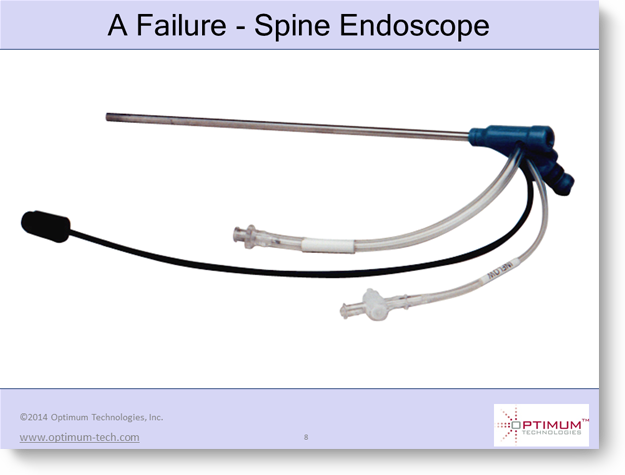 This single-use spine endoscope for percutaneous discectomy was developed at the request of one of the biggest medical device companies.
This single-use spine endoscope for percutaneous discectomy was developed at the request of one of the biggest medical device companies.
They were convinced that this device not only had to be sterile every time, but must also be pyrogen free, i.e., does not contain contaminants that can cause a fever response.
Such contaminants can be present on reusable devices even after proper reprocessing, so they mandated that this device be single-use.
All of the engineering specs for the device. The target production cost. And then the device was a spectacular failure.
It turned out that the marketing was just plain wrong. The device did NOT have to be pyrogen-free.
So the $900 disposable cost was not accepted by clinicians, when a high-quality, reusable spine scope could be reprocessed for a small fraction of that cost. And, by the way, the reusable had much better image quality because the manufacturing cost could be so much higher.