Client Type
Startup Medical Device OEM (TearScience, Inc.)
Unmet Need
A quantitative instrument for the diagnosis of ocular tear film deficiencies and response to therapy.
Approach
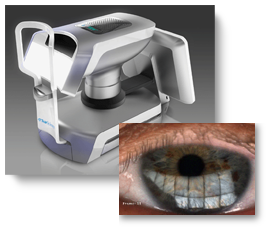
Starting with a prototype instrument developed by the optometrist-inventor and an early stage engineering team, develop the analysis algorithm and associated optics to process the resulting tear film image quantifying the results.
Product Features
This device creates a new category of ophthalmic instruments. Over 20 million Americans suffer from dry eye, a deficiency of natural oils (lipids) that lubricate the cornea. Until now, there has been no commercial device that can quantitatively assess the human tear film for the diagnosis of dry eye and other ocular syndromes. Using principles of white light interferometry, this new device illuminates the anterior of the subject’s eye and captures high resolution color video images of specular reflections from the cornea. The lipid and aqueous layers on the cornea modulate reflected colors, much like an oil slick on water. The advanced analysis algorithm eliminates blinks, subtracts the image of the iris in the background, correlates reflected color modulations to lipid thickness, and produces a report quantifying the tear film. The device is being presented at the ARVO meeting in May, 2010. The device has received 510(k) clearance.
Services Provided by OTI
- Measurement theory and algorithm development
- Optical design & engineering of illumination and imaging systems
- Prototype fabrication
- Extensive design verification and validation protocol development and testing
- Development of phantoms and calibration methods
- Technical support of 510(k) application